Background: Hodgkin lymphoma (HL) is a lymphoid neoplasm with high cure rates. Around 90% of patients (pts) will achieve response to first-line treatment. Real-world studies in Latin America (LATAM) are lacking. Moreover, inequity in the access to imaging technology and drugs represent a real challenge for Latin American countries with palpable influences in patient outcomes. Herein, we describe the clinical features, treatment patterns, and outcomes of HL pts managed in LATAM.
Method: We conducted a retrospective cohort study of adults aged ≥17 years with newly diagnosed HL across academic centers in 7 LATAM countries from 2003 to 2022, with follow-up through July 2023. Medical records were manually reviewed, and data were abstracted in a standardized form. Cancer staging was performed by Ann Arbor and German HL Study Group criteria. Survival probabilities were estimated with the Kaplan-Meier method and compared with the Log-rank test. Multivariable Cox regression models were fitted by cancer stage stratification. A landmark analysis was performed to assess the lack of PET scan availability at the end of treatment (EOT) in LATAM.
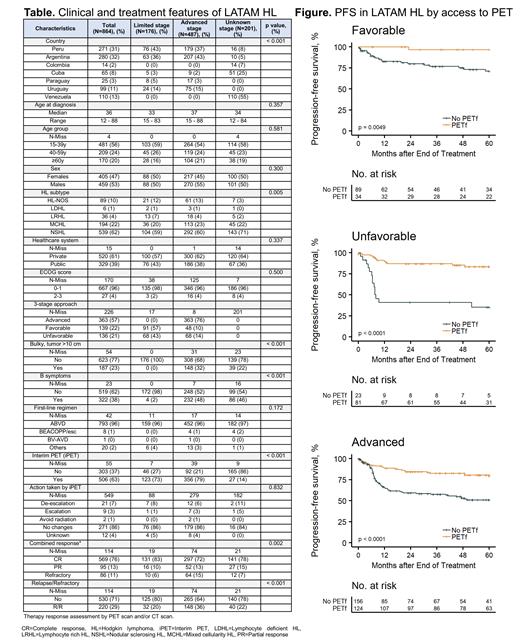
Results: Of 965 pts identified, 864 had sufficient data for analysis. Pts were young (56% <40 years; median 36 [17-88]) with slight male predominance (53%). 9 (3%) pts were HIV positive. Nodular sclerosing (62%) and mixed cellularity (22%) were the most common HL subtypes (p<0.01). Clinically, most pts had good performance status (ECOG≤1, 96%), no B symptoms (62%) and normal serum LDH (60%). Mediastinal presentation was seen in 14%, bulky mass (>10 cm) 23%, nodal involvement >3 sites 34% and extranodal involvement 25%. Advanced stage was common (57%); 22% and 21% had favorable and unfavorable limited stage HL, respectively. ABVD was the most common first-line regimen (96%); 8 pts received BEACOPP, and 1 BV-AVD. Radiation was utilized in 40% of limited and 21% of advanced stage HL. Only 63% and 48% had interim (iPET) and EOT PET scan, respectively. Most pts were managed at private than public institutions (59%, p<0.01). With a median follow up of 65 [59-71] months the 5-yr overall survival (OS) and progression-free survival (PFS) rates in all HL pts were 85% (82-88, 95% CI not reached, NR) and 64% (60-69, 95% CI NR), respectively. Better OS and PFS were seen in pts younger than 60 (p<0.01), ECOG≤1 (p<0.01) and limited stage HL (p<0.01). Overall response rate at the EOT assessed by either PET or CT scan was 89% (76% complete and 13% partial). In those assessed by iPET, results yielded 86% keeping same plan, 7% de-escalation and 3% escalation. In our cohort, 11% had refractory HL and 18% relapsed after achieving response. Given the inferior PFS to first-line seen in our LATAM HL pts compared to historical cohorts, we looked at possible factors associated to early relapse. Those assessed by PET at the EOT had significantly superior 5-yr PFS than those assessed by CT scan (PET vs CT: favorable HL 91 vs 71%; unfavorable 83 vs 35%; advanced 79 vs 51%) ( Figure). OS was inferior only in pts with unfavorable HL not assessed by PET (94 vs 76%, p=0.03). In the multivariable analysis, the lack of PET assessment at the EOT was associated with short PFS in unfavorable (aHR 7.81 [1.46-41.88], p=0.02) and advanced (aHR 17.35 [4.66-64.61], p<0.01), and a non-statistically significant worse PFSvin favorable HL (aHR 5.05 [0.56-45.72], p=0.15). Other factors associated to short PFS were extranodal disease (aHR 35.29 [5.62-209.07], p<0.01) in unfavorable, and high serum LDH (aHR 2.31 [1.2-4.46], p=0.01) in advanced HL. Interestingly, pts with advanced HL managed in public institution had less risk for relapse than those in private institutions (aHR 0.13 [0.03-0.51], p<0.01).
Conclusion: To our knowledge, this is the largest cohort of newly diagnosed HL pts in LATAM in the real-world setting. We observed similar clinical features in LATAM HL pts than those previously reported. ABVD was widely utilized in LATAM, and the 5-yr OS of 85% in all pts aligns with international estimates. However, our findings underscore the impact of limited access to PET scan at the EOT in LATAM, leading to lower PFS outcomes compared to those reported in developed countries. Despite this challenge, salvage therapy seems to rescue our LATAM HL pts, thus, OS remains optimal. To improve outcomes and minimize late effects following treatment completion, increasing the use of PET-adapted therapy for managing adult pts with HL in LATAM should be prioritized.
Disclosures
Quintero:Merck Sharp and dome: Speakers Bureau; Takeda: Speakers Bureau; astra zeneca: Speakers Bureau; roche: Speakers Bureau. Castillo:Loxo: Consultancy, Research Funding; Cellectar: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Kite: Consultancy; Mustang Bio: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal